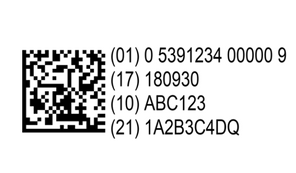
The Falsified Medicine Directive is an international solution for the issue of counterfeit medicines. Designed to eradicate falsified medicines, the Directive is introducing technology to allow pharmacists to identify and prevent counterfeit medicines from entering the supply chain. The FMD means that the following two safety features must be on all new packs of prescription medicines placed on the market in Europe after February 9th, 2019: - A unique identifier (UI) in the form of a 2D barcode which can be scanned at various points during the supply chain to determine authenticity. Almost all prescription medicines will require scanning authentication prior to dispensing.
- An anti-tamper device on the outer packaging of medicines.
|
| The unique identifiers, in the form of a 2D barcode, means that manufacturers will be able to load their products into the database (which is held centrally by the European Medicines Verification Organisation) so that they can be recognised and authenticated by the pharmacist by scanning the barcode upon dispensary. Medicines which have been decommissioned will also be held in the database, enabling a warning alert to show if the medicine is faulty or has been stolen. |  |
| While entire supply chain will be affected by the new legislation, pharmacies will be most affected as they will need to adapt in order to check the compliance of the medicine that they sell and ensure that they are adhering to the Directive. This adaption will involve the introduction of scanning technology to allow pharmacists to determine the authenticity of the medicine. The pharmacist will need to scan the 2D barcode on each packet communicating with the National Medicine Verification System to change the status of the pack from ‘active’ to ‘inactive – supplied’. This will involve the large step of implementing scanning technology at the point of dispensing, a technology which many pharmacists lack. |
| Zebra scanning technology helps pharmacies to achieve compliance by enabling them to scan, record and verify all medications that the hold on stock and dispense to patients. Compliance with the Falsified Medicines Directive and the introduction of scanners to the workplace will enable pharmacies to improve their stock management processes and prevent falsified medicines from entering the supply chain. |
 |
We hold a range of FMD compliant Zebra barcode scanners and smartphones (features including 2D scanners) which can be found at the following links: For any questions about FMD compliant scanners please contact us on 01978 437537 to discuss your requirements. |
 |
 |